What Does Sulfuric Acid and Calcium Fluoride Make
Calcium sulfate CaSO 4 is removed through an air lock at the opposite end of the kiln and the gaseous reaction productshydrogen fluoride and excess sulfuric acid from the primary reaction and silicon tetrafluoride SiF 4 sulfur dioxide SO 2 carbon dioxide CO 2 and water produced in various secondary reactionsare removed from the front end of the kiln. This is because only the hydrogen and fluorine atoms would rearrange to form hydrofluoric acid.

How To Balance Ca H2so4 Caso4 H2 Calcium Sulfuric Acid Youtube
The reaction mixture comprises sulfuric acid and suspended solids.

. The shallow hole shrinkage method is used in the mining process. Vinegar acid breaks apart the solid calcium carbonate crystals base in the eggshell into their calcium and carbonate parts. The experiment generally uses calcium carbonate and hydrofluoric acid or repeated treatment of fluorite powder with concentrated hydrochloric.
The calcium ions stay dissolved in the vinegar calcium ions are atoms that are missing electrons while the carbonate goes on to make carbon dioxide the bubbles that you see. Calcium Fluoride is relatively inert and non-hygroscopic. The calcium carbonate will dissolve in the acid producing CO2 gas.
The chemical reaction which produces gaseous hydrogen fluoride and solid. How does sulfuric acid absorb water. Ammonium sulfate is a ubiquitous agricultural.
The solids comprise unreacted starting material especially calcium fluoride and in. HF is manufactured by reaction of the mineral fluorospar calcium fluoride with sulfuric acid in the presence of heat. What does calcium hydroxide and sulfuric acid make.
The reaction between calcium and sulphuric acid produces calcium sulphate a salt and hydrogen gas. The balanced equation for this reaction is. For example mixing starch C 6 H 12 O 6 n and concentrated sulfuric acid will give elemental carbon and water which is absorbed by the.
CaF 2 H 2 SO 4 CaSO 4 solid 2 HF. It is a single displacement reaction. The balanced equation will be.
How does caco3 neutralize acid. Insoluble in water slightly soluble in inorganic acid. It is extremely poorly soluble in water 00016 g100 mL at 20 C and insoluble in organic solvents.
The HF is then dissolved in water to form hydrofluoric acid. 1 Answer to Solid calcium fluoride CaF2 reacts with sulfuric acid to form solid calcium sulfate and gaseous hydrogen fluoride. Does caco3 dissolve in acid.
And hydrofluoric acid formed by the action of hot concentrated sulfuric acid. Fluoride ions migrate through the body destroying tissue until lodging in. A source of calcium fluoride is fluorite ore.
Double Replacement Reaction. Calcium fluoride react with sulfuric acid to produce hydrogen fluoride and calcium sulfate. Hydrofluoric acid HF is produced by the reaction of concentrated sulfuric acid with fluorspar calcium fluoride.
Industrial chemists make hydrofluoric acid which is used in aluminum and uranium processing to etch glass and to make CFCs from the reactions of. Calcium fluoride will react with a strong acid such sulfuric acid to yield calcium sulfate and hydrogen fluoride. We could isolate the salt ammonium sulfate N H 42SO4 from such a solution.
H2SO4 aq CaOH2 s CaSO4 s 2H2Ol If 3. The action of strong sulfuric acid liberates hydrogen fluoride from the mineral. As a fluoride source calcium fluoride in the fluorite state is of major commercial relevance.
Altough HCl is a stronger commercial acid than sulfuric as measured by its pKa the industrial common way to produce HF is to treat fluorspar calcium fluoride aka fluorite with sulfuric acid obtaining gypsum as by-product. 11302018 - 0759. Answer 1 of 4.
H 2SO4aq 2N H 3aq 2N H 4 SO2 4. Sulfuric acid is produced from sulfur oxygen and water via the contact process. The affinity of sulfuric acid for water is sufficiently strong that it will take hydrogen and oxygen atoms out of other compounds.
Colorless crystal or white powder. Why does calcium stop reacting with sulfuric acid. HCl is used because the line is of pvc make.
Flourine is used to make hydrofluoric acid and hydrofluoric acid was invented in the late 1700s. When sulfuric acid reacts with calcium hydroxide calcium sulfate and water are produced. The calcium sulphate is insoluble in dilute sulphuric acid and so you end up with calcium carbonate coated in a layer of calcium sulphate which stops the acid from getting at the carbonate and so stops the reaction.
Heating the reaction mixture to the temperature of 130-200C 8 200-250C 17 The technical method production hydrogen fluoride. To add a bit of information to the excellent explanation by Kenvlach. Ca H2SO4 CaSO4 H2.
It produces calcium sulphate and hydrogen fluoride. The rest of the atoms of the reactants would have to rearrange to form another substance. N H 3aq H 2Ol N H 4 H O but the equilibrium lies to the left and the dominant species in solution is N H 3aq.
How is sulfuric acid manufacture. H2SO4 CaOH2 CaSo4 2H2O One molecule each of sulfuric acid and calcium hydroxide react to give one molecule of calcium sulfate and TWO molecules of water. Mixed the sulfuric acid and the calcium fluoride to make hydrofluoric acid then the other product of this reaction could be calcium sulfate.
The reaction can take place in two stages. Calcium fluoride chemical formula CaF2. What salt does hydrofluoric acid with barium hydroxide make.
The neutralization process occurs when strong acids in intimate contact with limestone chips react with Calcium Carbonate CaCO3 the primary constituent of limestone to form water carbon dioxide and calcium salts. Calcium fluoride is a white solid compound. And so we could rewrite the reaction as.
Single crystals are transparent. CaF 2 H 2 SO 4 2 HF CaSO 4 Physical.

How To Balance Caf2 H2so4 Caso4 Hf Calcium Fluoride Sulfuric Acid Youtube
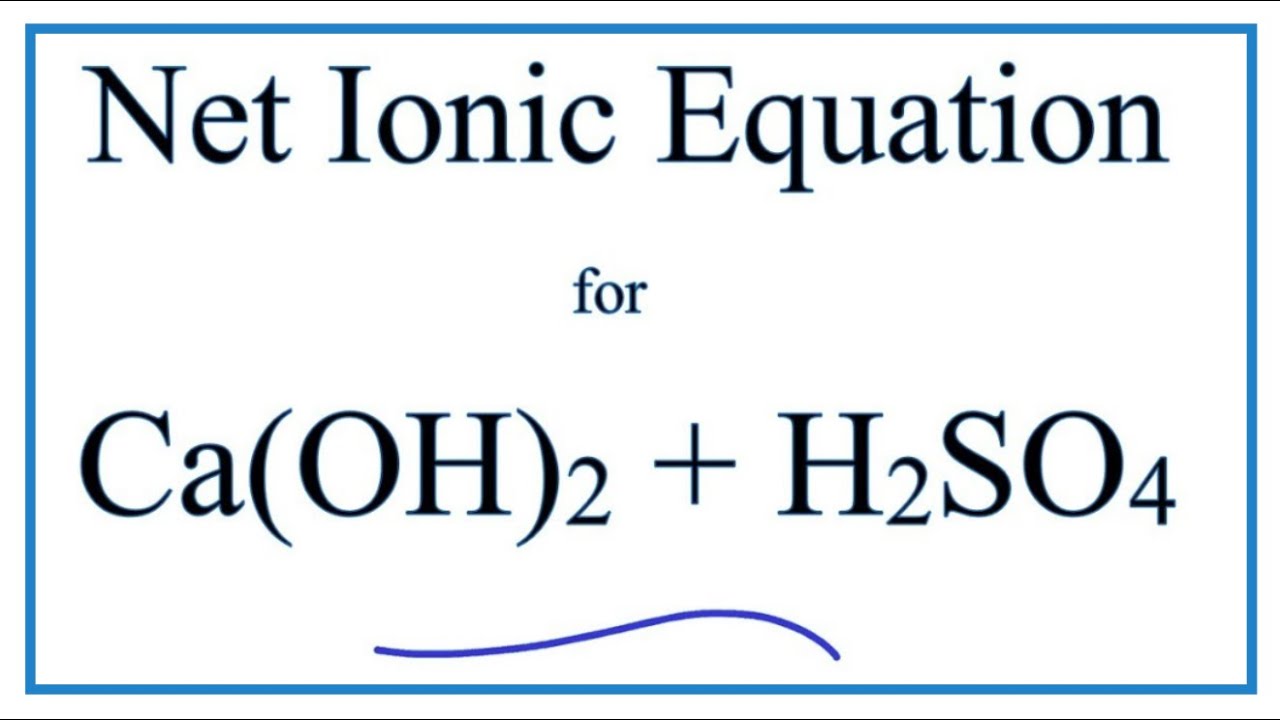
How To Write The Net Ionic Equation For Ca Oh 2 H2so4 Caso4 H2o Youtube

How To Balance Caf2 H2so4 Caso4 Hf Calcium Fluoride Sulfuric Acid Youtube
No comments for "What Does Sulfuric Acid and Calcium Fluoride Make"
Post a Comment